This toolbox describes in detail technical and logistical measures that may be necessary in biological emergencies.
The technical measures include
-
Diagnostics
-
Occupational safety and health/personal protective equipment (PPE)
-
Surveillance
-
Disinfection measures
-
Protective measures
-
Isolation
The logistical measures include:
-
Patient transport
-
Sample management
-
Therapy and vaccination strategy
-
Handling of corpses
-
Disposal management
The information contained here is of a generic nature. For numerous pathogens there are specific recommendations, which can be found e.g. on the RKI website under Infektionskrankheiten A-Z (Communicable diseases A-Z), and on the websites of the federal state authorities or where appropriate the respective competent authorities.
Technical measures
Diagnostics
Diagnosing the disease is an essential part of assessing which therapeutic and epidemiological measures are necessary. Diagnostics encompasses taking the patient's case history, performing a physical examination and performing examinations using apparatus, including laboratory analysis. In many communicable diseases, diagnosis is made more difficult by the fact that when the disease begins the symptoms are often so unspecific that laboratory tests are needed in order to confirm the diagnosis.
Accordingly, the requirements for laboratory diagnostics are as follows:
-
High sensitivity: it must be possible to detect minimum amounts of a pathogen, in order to avoid false negative results
-
High specificity: it must be possible to clearly distinguish between similar pathogens/antigens, in order to avoid false positive results
-
High stability: minimum effect of factors that might falsify the result by producing false positives or false negatives (e.g. inhibitors)
-
High rapidity: shortest possible time until test result is available, so that the right measures can be taken as quickly as possible
Optimal diagnosis requires proper sampling, as well as the right packaging and shipment (also described in this toolbox). In order not to lose any unnecessary time in a crisis, the teams managing the crisis should be aware of corresponding laboratories within their sphere of responsibility. They should also know what range of diagnostic services the laboratories provide and have their contact details. To help make the process smooth one it is a good idea to discuss something with the laboratory – and in particular to determine which type of samples are most appropriate. The laboratory should also be notified in advance so that it can begin preparatory measures before the sample arrives.
At the beginning of an outbreak or in the case of diseases with high lethality, it is necessary to diagnose every single case. In the course of an epidemic, however, overstretched laboratories or clear epidemiological links may necessitate diagnosis based on case definitions, which are actually designed to ensure standard criteria for the surveillance of communicable diseases Germany-wide.
Occupational safety and health/personal protective equipment
Basics
In Germany, work with biological agents is governed by the Biological Agents Ordinance (BioStoffV), which aims to ensure safety and health protection at workplaces. The Committee on Biological Agents (ABAS) at the Federal Ministry of Labour and Social Affairs uses the Ordinance to determine the state of the art in science, technology, occupational medicine and occupational hygiene, and makes this information available in the form of Technical Rules for Biological Agents (TRBA).
These cover:
-
General information, structure and application
-
Activities involving biological agents (e.g. protective measures in laboratories, in acute biological emergencies, solid waste, waste water, health care)
-
Assessing hazards (e.g. classification into risk groups)
-
Hygiene and disinfection measures
-
Decisions of the ABAS on requirements for activities involving biological agents in special cases (e.g. tuberculosis, polio, influenza, highly pathogenic agents).
The TRBAs are designed to support the optimal implementation of occupational safety measures. They are not statutory regulations. It is therefore possible to apply other measures that have the same effect on safety.
Occupational safety and health operates on the principle that structural measures should be considered before organisational measures, and organisational measures before personal measures. Accordingly, a risk assessment should be performed and appropriate protective measures defined prior to any activity. The principle of maximum protection need not be applied in all cases, as this would place an unnecessary burden on the institution, and in the worst case might even contribute towards patients not receiving optimal care.
Personal protective equipment (PPE)
The TRBAs describe the requirements for personal protective equipment (PPE), e.g. in health care and welfare facilities (TRBA 250) or for the care of patients infected with highly pathogenic organisms outside of special isolation units (Resolution 610). The TRBAs list numerous standards that the PPE must meet. When procuring PPE it is advisable to require the manufacturer to meet the specified standards, as this will guarantee compliance with the scientific state of the art.
Protective equipment is manufactured and classified in accordance with Directive 89/686/EEC (Council Directive of 21 December 1989 on the approximation of the laws of the Member States relating to personal protective equipment). For protection against mortal hazards or serious irreversible injury, category III is used.
When selecting PPE, a basic distinction can be drawn between the infection protection set, which comprises various components, and a protective (hazmat) suit with powered air-purifying respirator, which often comprises a one-piece protective garment with an external blower.
The advantages of the infection protection set are listed as the low price per set and low maintenance requirement. The advantages of the protective suit are the greater comfort, longer wearing times, greater ease of communication when using a headset, no unpleasant odour on decontamination (when using ABEK P3 filters). Although the error rate when donning the infection protection set is higher than with the protective suit, both systems require regular training. Errors regularly occur when doffing PPE that can have life-threatening consequences in an emergency.
We will now discuss these suit components individually. For full descriptions please refer to TRBA 250 and Resolution 610.
Respiratory protection
The respiratory tracts are protected by filtering face pieces (FFPs). These are tested to DIN EN 149:2001 for impermeability to particulate matter and liquid aerosols. The class indicates the filtration efficiency:
-
FFP1 maximum 22%,
-
FFP2 maximum 8% and
-
FFP3 maximum 2% total leakage
As leakage decreases, protection increases, but so too does the strain caused by difficulty breathing. An exhalation valve will reduce the strain, and should therefore be selected particularly with FFP3 masks. The protective effect can also be improved by a correct fit. Since people's heads are different shapes, each individual needs to test which mask is most suitable for them. For this purpose a FIT test should be carried out. If the wearer has a beard or heavily scarred skin in the area around the sealing lip, the mask will not fit tightly. To guarantee additional splash protection the filter fleece should also be tested to DIN EN 14683 (splash protection IIR).
The surgical face masks often worn to cover the nose and mouth do not provide respiratory protection. These should be used primarily by sick people who are healthy enough to use them to prevent the spread of pathogens. A respirator mask with exhalation valve, on the other hand, should not be used on sick people, because these masks create strong resistance to inhalation and have no filtering effect on exhalation.
When using a full-face mask or a protective suit with blower unit, filters can be selected that also protect against gases/chemicals. The Deutsche Gesetzliche Unfallversicherung (German Social Accident Insurance Company – DGUV) has put together some important advice on using respirators.
Eye protection
When selecting eye protection as part of an infection protection set it is important to make sure it matches the other components. One especially crucial aspect is the junction between the respirator mask and the eye protection. In protective suits with blower units the eye protection is integrated. Depending on the risk assessment, full goggles may be required that are fully-enclosed, non-vented and anti-fog. If there is no risk of aerosol formation, a visor can also be used. The testing criteria are defined in EN 166. According to this standard, the protective goggles should have frame mark 5.
Hand hygiene
Since most pathogens are transmitted by hands, hand protection is especially important. Here we should also emphasise that gloves can also transmit biological agents. Wearing gloves does not exempt the wearer from practising hand hygiene. When choosing gloves, both mechanical and biological protection should be considered.
It is important to hold discussions within the municipality/the district and with the other authorities, and to work out a uniform strategy for PPE, decontamination and training.
Need for protective equipment
To be able to calculate the consumption of PPE it is necessary to consider both the nature and the severity of disease, as these will determine both the type of PPE required as well as the frequency of contact with the various occupational groups. The American Centers for Disease Control and Prevention have published a clearly structured calculator tool on their website. Click here to see examples of the PPE requirement in five different scenarios:
Estimated Personal Protective Equipment (PPE) Needed for Healthcare Facilities.
N.B.: The most important form of personal protection is to have the vaccinations available! A risk analysis must be performed with the company doctor.
Surveillance
Epidemiological surveillance is
"the continuous and systematic collection, analysis, evaluation and dissemination of health data for the purpose of planning, implementing and evaluating disease control measures" RKI, 2020 (n.d.).
Surveillance is designed to ensure early detection and prevention of the spread of infections.
Chapter Three of the Protection against Infection Act lays down the legal foundations for notification in Germany. It defines: the notifiable diseases, the notifiable evidence of pathogens and the persons obliged to notify, as well as the channels of communication from the health office via the federal state authorities to the federal authority and the World Health Organization. In crises the Federal Ministry of Health (BMG) is empowered by Section 15 to terminate, restrict or extend the obligations to notify, in the so far as permissible or required according to the epidemiological situation, by issuing an ordinance.
Protective disinfection measures
Since pathogens can often be transmitted via animate and inanimate services, it is necessary to regularly disinfect all objects with which an infectious person may have come into contact, in order to prevent the spread of pathogens. In particular it is necessary to ensure final disinfection when a patient leaves a place, such as an ambulance or a room following their discharge, in order to protect persons who subsequently enter those places. With diseases transmitted by aerosols, it may also be necessary to carry out room disinfection in addition to scrubbing and wiping down services.
Disinfection measures also apply to persons who may have been contaminated by an infectious individual or objects. Hand hygiene is especially important in this context because most diseases are transmitted via contaminated hands. While it is often sufficient in the home environment to wash hands thoroughly, in areas with dangerous pathogens, such as in medical facilities or diagnostic laboratories, hand disinfection is mandatory in order to protect staff, patients and visitors.
Section18 of the IfSG stipulates that for disinfection measures ordered by an authority, only agents and procedures may be used that have been published by the competent higher federal authority, in this case the Robert Koch Institute, in the Federal Health Gazette. For disinfection, a distinction is drawn between thermal, chemical and other methods. When selecting a suitable disinfectant it’s necessary to consider the spectrum of action of the various agents. Put simply, a distinction is drawn between the following categories:
A. Disinfectants that kill vegetative bacteria.
B. Disinfectants that inactivate viruses; a 'virucidal' disinfectant is active against both enveloped and non-enveloped viruses, while a disinfectant of 'limited virucidal activity' acts primarily against enveloped viruses.
C. Disinfectants that kills spores of the anthrax pathogen.
D. Disinfectants that kill spores of the gaseous oedema and tetanus pathogens.
The RKI publishes a list of tested and recognised agents and methods. Since professional disinfection requires a high level of specialist expertise, the Commission for Hospital Hygiene and Infection Prevention has published a recommendation on staff and organisational requirements for the prevention of nosocomial infections.
The list of disinfectant agents published by the Association for Applied Hygiene is the standard reference for routine disinfection in medical and non-medical facilities. While decontamination is usually understood to mean the reduction of harmful agents to a level that is not harmful to health, disinfection means the killing or irreversible in activation of pathogens. Consequently, unlike disinfection, can mean only mechanical removal without inactivation/killing (see also: Decontamination of affected persons).
Protective measures
Post-exposure prophylaxis
With some infectious diseases there is a basic possibility that initiating prophylaxis immediately following an exposure/possible infection may prevent the outbreak of a disease.
Examples of diseases that can be prevented with antibiotic post-exposure prophylaxis (PEP) include:
-
Meningococcal meningitis
-
Anthrax
-
Tularaemia
-
Plague
Some viral diseases can also possibly be prevented by giving individuals PEP. These include:
-
Viral haemorrhagic fever
-
Smallpox
-
Influenza
Where infection is suspected, the physician should consider whether PEP is possible and whether it is indicated.
The public health service should conduct up-to-date risk assessments for its own area of responsibility. This includes answering the following questions:
-
Which communicable diseases must (realistically) be expected?
-
For which of these diseases can medicinal PEP be considered?
-
Which medicines are suitable as PEP?
-
Will sufficient quantities of these medicines also be available to the public health service in crisis situations?
-
Would it make sense for the health service to stockpile medicines itself? Does the health service know what stocks neighbouring health offices might keep?
-
Is there a plan for distributing PEP?
-
Will the resources for distributing PEP also be available in a crisis?
-
Have information and documentation materials been prepared in case of PEP?
-
Is post-exposure vaccination possible, and does it make sense?
-
Are the required vaccines available in sufficient numbers?
-
Are materials for transporting, storing and administering the vaccines available?
-
Is there a plan for administering post-exposure vaccinations?
-
Have information and documentation materials been prepared in case of post-exposure vaccination?
Isolation/quarantine
The purpose of isolation/quarantine is to reduce or if possible prevent the spread of pathogens. See also the dictionary of specialised terms in infection control (German only).
The IfSG describes the pertinent measures systematically in Sections 28 and 30.
Pursuant to Section 30 IfSG, quarantine is an isolation measure for persons who are ill, suspected of being ill, suspected of being contagious or germ carriers. This can take place in an appropriate hospital or by any other appropriate means. Exceptions may be made for germ carriers, provided that they comply with other protective measures.
In contrast to quarantine, pursuant to Section 28 IfSG isolation measures are possible that force persons not to leave the place they are in or not to enter places specified. This might also include isolation in the home, for instance.
The practical aspects of isolation measures
Experience with outbreaks in recent years where domestic isolation was ordered, for instance, show that those concerned often lacked understanding of why the measure was meaningful. In some cases the individuals concerned saw it merely as a 'ban on going to work', believing that although they were not supposed to leave their homes, they could receive visitors.
It is therefore advisable to issue the order to the individuals concerned in writing, and draw their attention to the consequences of failure to comply. Violations are punishable with a fine or imprisonment.
It is important to provide those concerned with recommendations on how they should behave in the home environment, especially when other persons are present in the same dwelling who have not been told to isolate. These include hand hygiene and mutual distancing. Particularly for families with small children this can pose a major challenge.
Providing food to isolated persons who have no neighbours or relatives to do so can also be a particular challenge. For this the public health office can prepare a list of service offerings, e.g. delivery services provided by grocery shops or pharmacies.
Mental health is also an important component, as fears, worries about infection and loneliness, for example, can contribute to people in isolation failing to comply with the official order or abandoning any attempt to do so. So, inform those in isolation about ways of keeping themselves occupied and fit, how they can stay in touch with others and who to contact in an emergency.
Logistical measures
Patient transport by the emergency services
In biological emergencies, transporting patients creates particular challenges for the emergency services.
As a rule, the aim should be to transport patients once they have been decontaminated. The official fire service regulation 'Units deployed on CBRN operations' (FwDV 500) states in this connection:
'If medically justifiable, contaminated casualties should be decontaminated under the responsibility and direction of the emergency services (emergency doctor). (...) With some CBRN dangerous substances that would cause significant further damage if contamination were to spread (e.g. warfare agents, especially bioweapons and infectious substances), decontamination/disinfection at the scene is required.' (AFKzV 2012)
In chemical, radiological and nuclear emergencies, transporting non-decontaminated patients can cause serious problems down the line, As well as endangering personnel and the public, contamination may also be spread in vehicles and thereafter in health care facilities. In the worst case scenario, in the course of a crisis this may lead to a failure of this infrastructure.
Transport of infectious persons
The transport of infectious patients by the emergency services usually follows a different logic than in chemical and R/N emergencies. Since the patient continuously excretes the pathogen, decontamination often does not make sense. Transport under these conditions presupposes certain protective measures. For this purpose, the actors of the emergencies services draw up hygiene plans. These should be produced in collaboration and consultation with the responsible persons at the public health office.
The scope of the needed protective measures must be based on a classification of the patient. The State Institute for Work Design in North Rhine-Westphalia has published information (German only) on infection risks in the emergency services.
Decontamination of contaminated persons
With persons externally contaminated with pathogens, on the other hand, it is advisable to decontaminate the patient before transporting them, in order to prevent spread of contamination. One example would be transporting an individual who has come into contact with an unknown biological substance. This patient should be decontaminated. It can be assumed that a large part of the contamination can be removed simply by removing the patient's clothing. The body should then be cleaned mechanically with plenty of soap and water. If possible the shower water should be collected and sent for separate decontamination (e.g. chemical or thermal). A decontaminated person should nevertheless continue to be seen as potentially infectious.
The RKI has also published specific information on what to do when powder is discovered.
Transport capacities in a crisis
When preparing for a crisis it is necessary to find out from the responsible emergency services which protective measures will be implemented, and what kinds of patient can be transported safely and in what numbers.
When calculating the availability of emergency equipment it is also necessary to take into account downtimes for disinfection. You should also think about staff shortages during an outbreak.
-
What capacities for transporting infectious patients can the responsible emergent services realistically provide?
-
What other capacities can be mobilised, e.g. assistance from outside the locality, and within what time frame?
-
Have notification channels and responsibilities been defined?
Patient isolation and transportation, transport of highly infectious patients
Under normal circumstances it will not be possible at the local level to transport patients with life-threatening, highly infectious diseases safely. These diseases include:
-
Pneumonic plague
-
Smallpox
-
Viral haemorrhagic fever
The mere suspicion of one of these diseases should lead to the patient being transported with appropriate protective measures. The destination will usually be a high-level isolation unit (HLIU) or biocontainment unit (BCU).
To isolate and transport such highly contagious patients using HLIUs deployed by transporters, or patient isolation units (PIUs), special vehicles (infectious disease ambulances), specially trained personnel and special protective equipment are kept at individual locations in Germany. There is no single standard, however. Given the many enquiries received by the Robert Koch Institute, we would like to point out that for the primary protection of staff, the crucial element is compliance with protective measures such as the correct use of PPE, rather than the means of transport. The advantage of deployable HLIUs and PIUs is that it is easier to decontaminate the vehicle because of its smooth surfaces. If a conventional ambulance is used, medical equipment that is not required should be removed beforehand if possible, as this is difficult to decontaminate and the manufacturer usually cannot guarantee that it will continue to work properly after decontamination.
Transporting patients in this way is logistically complex, and considerable time is required in advance. The number of possible deployment is very limited. Information on the locations where deployable HLIUs/PIUs are available is kept by the Permanent Working Group of Competence and Treatment Centres for high consequence infectious diseases (STAKOB).
Samples
Sampling
A basic distinction needs to be drawn between clinical sampling and environmental sampling. We will not deal with clinical sampling in any further detail below, as this is part of daily routines. Should an unusual clinical case arise that necessitates special sampling, the experts on the RKI's STAKOB are available to give advice.
In the case of environmental contamination too, professional sampling is key to successful sample analysis. Before sampling, an agreement should be reached with a suitable laboratory as to what sample should be taken, how, and in what quantity. You should also make sure that the laboratory can process environmental samples. If a (bio)terrorist attack is suspected, in which the perpetrators also need to be identified, it is also necessary to work closely with the competent police authorities in order to avoid destroying important evidence. Trained personnel may also be required to take the samples. It is not automatically to be assumed that every public health office will be able to do so right away.
Unlike chemical, radioactive and nuclear substances, the real-time detection of biological agents has not yet proved sufficiently reliable. Although they are continuously improving, commercially available rapid tests for environmental analysis continue to produce too many false positive and false negative results
As the samples are taken in a contaminated environment, the personnel taking the samples must also protect themselves (see Occupational Safety and health/PPE and TRBA 130). Before they enter the contaminated area, arrangements also need to be in place for subsequent decontamination when they leave the sampling site. If the public health office does not wish or is unable to perform sampling itself, it should make advance arrangements with the local fire services. The BBK has also published further information in its Recommendations on sampling for hazard prevention in population protection. These describe sampling in cases of chemical, biological or radioactive contamination. The European Commission has also published a guideline on Biological incident response & environmental sampling.
Transport of samples
The transport of dangerous goods – which include CBRN samples – is regulated by the European Agreement concerning the International Carriage of Dangerous Goods by Road (Accord européen au transport international des marchandises Dangereuses par Route, ADR). The ADR is revised every two years, so is important to make sure you are using the current version.
Who is responsible
Dangerous substances are separated into different dangerous goods classes, for which specific labelling, packaging and transport regulations are prescribed. According to Section 18 of the German Ordinance on the Transport of Dangerous Goods by Road, Rail and Inland Waterways (GGVSEB) the consignor is responsible for compliance with the pertinent transport regulations. In case of doubt this will be the head of the consigning institution, therefore e.g. the director of the public health office.
A detailed explanation of the proper preparation of shipments can be found in the World Health Organization's Guidance on regulations for the Transport of Infectious Substances:
-
Classification of the shipment
-
Correct packaging of the shipment
-
Correct labelling of the shipment
-
Original documentation for the shipment
-
Presentation of the required import permits, making of advance arrangements with the carrier to ensure that the shipment will be accepted for appropriate transport and that the shipment is undertaken by the most direct routing.
Classification
For chemical substances, depending on the nature and state of the substances
the following classes (in some cases with subclasses) apply
Class 2 - gases and gaseous mixtures
Class 3 – flammable liquids
Class 4 – flammable solids
Class 5 - oxidising agents
Class 6.1 – toxic substances
Class 8 – corrosive substances
For radiological/nuclear materials hazardous materials class 7 – radioactive materials – applies.
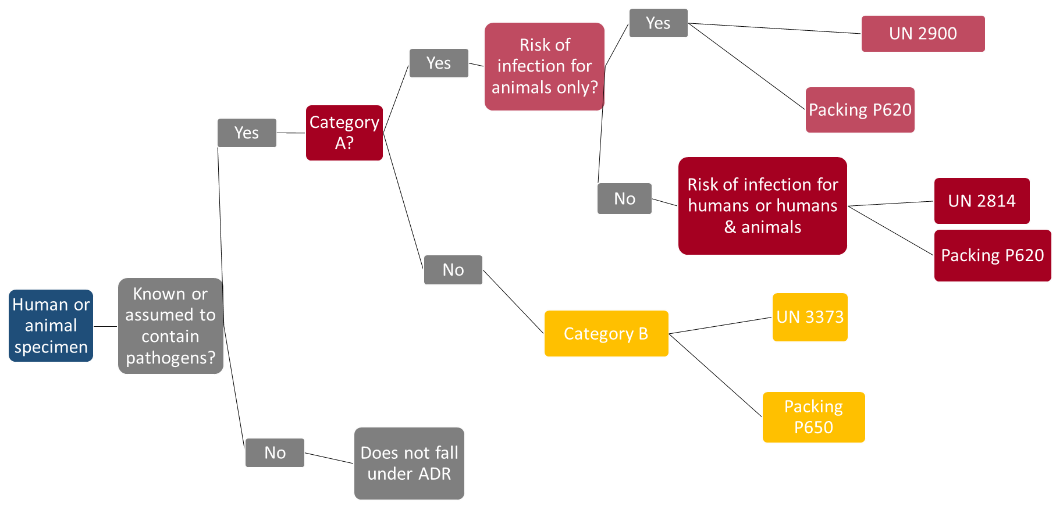
Biological materials fall under class 6.2 – infectious substances. In the intendment of the ADR, infectious substances are defined as substances which are known or are reasonably expected to contain pathogens.
Biological specimens are further subdivided as follows:
-
Class 6.2 Category A: An infectious substance which is transported in a form that, when exposure to it occurs, is capable of causing permanent disability, life-threatening or fatal disease in otherwise healthy humans or animals. A list of the pathogens can be found in Annex 2 of the
-
World Health Organization's Guidance on regulations for the Transport of Infectious Substances. For Category A pathogens, hospital waste also falls under the classification UN 2814 or UN 2900 and the packing instruction P 620.
-
Class 6.2 Category B: An infectious substance which does not meet the criteria for inclusion in Category A. (classification: UN 3373; packing: P 650).
Exempted medical specimens: Specimens that do not contain infectious materials, for which there is minimal likelihood that they will cause disease in humans or animals, are not subject to the stipulations of the ADR, unless they meet the criteria for inclusion in another class. This includes e.g. non-pathogenic or inactivated micro-organisms, specimens that contain pathogens at naturally occurring concentrations (no UN number; packaging based on P 650).
Packaging instructions
Packaging comprises basically three components. The main differences lie in the different testing standards that the packaging must meet. The three components are:
-
leak-proof primary receptacle(s); surrounded by absorbent material in a quantity sufficient to accommodate the entire content
-
a leak-proof secondary packaging (except in the case of solid materials)
-
a rigid outer packaging.
Emergency transport
Exemption from the stipulations of the ADR is possible in case of emergency transport:
'Emergency transport intended to save human lives or protect the environment provided that all measures are taken to ensure that such transport is carried out in complete safety.' (Sub-section 1.1.3.1e ADR)
Emergency transport thus does not exempt from safe packaging. Some carriers offer to bring packaging along with them and pack the sample themselves. In such cases, arrangements should be agreed with carriers in advance.
In the case of primary samples in a bioterrorist attack or an acutely life-threatening disease, in which no vehicle is available that is officially marked for transporting dangerous goods and has properly trained personnel, a vehicle of the ambulance, fire or police can be used for emergency transport.
Measures to minimise contact
With infectious diseases that are transmitted via droplets and/or smear infection, the spread can be reduced by measures to minimise contact. These include above all strict hand hygiene, coughing and sneezing etiquette, and social distancing (1-2 m),
To apply contact-minimising measures on a targeted basis, it is advisable to identify relevant institutions and events.
Depending on the pathogen and vulnerable group, these can vary. Typical facilities include:
-
Schools / kindergartens
-
Nursing homes
-
Commercial kitchens
For major events such as trade fairs, concerts or sporting events, restrictions can be based on the following criteria:
-
Is it possible to introduce social distancing and other hygiene measures, or can the number of participants be reduced in order to make it possible? How well is the venue ventilated?
-
Can participants be screened at the entrance?
-
Can individuals displaying acute symptoms typical of the pathogen be excluded?
-
Can the event be postponed?
In this connection the RKI has published General principles for risk assessment and recommendations for major events.
Therapy and vaccination strategy
Among the most important preventive measures in the medical field are vaccinations. Since modern vaccines are very low risk and offer a very high degree of protection, it makes sense for medical personnel in particular to accept vaccination when offered so that they are protected in case of an outbreak. It may also be appropriate to vaccinate against other diseases, to prevent patients displaying similar symptoms from being suspected of having the disease. To protect the public high vaccination rates should be aimed for, as these will prevent the pathogen from spreading. In the case of human smallpox, very high vaccination rates have even succeeded in eliminating the disease altogether. This goal is currently being pursued for measles and polio.
With new pathogens it is to be assumed that a vaccine will not be available (immediately), and will first need to be developed. For vaccines that are already approved in principle and only need to be adapted to the current pathogen, this can be achieved within a few months (e.g. influenza). With other vaccines development can take months to years, followed by a longer approval process which is designed to ensure that the vaccine does not have any undesirable side effects.
Therapeutic strategies are available for numerous diseases. Even so, however, it is be assumed that there will be no known therapeutic options for emergent or very rare diseases, and that these can only be developed in studies in the course of the disease. The first patients can therefore only be treated by means of supportive therapy and, in a few cases, in individual therapeutic trials.
Consequently, the aim at the beginning of an outbreak of a disease for which no vaccine and no specific therapeutic agent is available, is to apply hygiene measures to delay the onset of an epidemic, until vaccines or medicines become available. For such eventualities, plans should be drawn up for delivering medicines or vaccines to the public as quickly and safely as possible. Plans of this kind have been drawn up Germany-wide for instance to ensure preparedness for an outbreak of human smallpox.
Handling of corpses
Unfortunately, in a crisis it will not be possible to prevent people from dying. This can pose a challenge for the public health service in two ways: firstly through a high number of corpses, and secondly through their contagiousness. Contingency plans should be drawn up for both emergencies. The relevant regional or national regulations should be observed.
High number of deceased
To be able to guarantee appropriate storage and burial even for a high number of deceased persons, responsible planners should ascertain whether and where there are cool rooms in the area where the remains of the deceased can be kept in a manner that is as ethical as possible, but without posing any risk to the public at large, until burial can take place.
Contagious corpses
With some diseases it is a known fact that the corpse still contains a high number of pathogens, and thus poses a risk to all individuals who have to deal with it. The key factors determining the infectiousness of corpses are the nature of the pathogen, the possible route of transmission and the viability of the pathogen in body fluids or in the body of the deceased through time. Internal autopsy should therefore be avoided if possible. If absolutely necessary, this should be conducted under conditions of safety level 3 or 4. The personnel required to perform the autopsy should be equipped with PPE, and should be trained in how to use it.
Religious and life philosophy wishes should in principle be respected. In the case of a highly pathological agent, however, it is generally advisable to avoid the following practices: ritual washing, laying out of the deceased, paying last respects at the open coffin and interment. Similarly, any implants such as pacemakers should not be removed, but must be borne in mind during cremation.
The deceased should be sprinkled in their entirety with a special absorbent to bind escaping body fluids. After that the deceased should be wrapped in two formalin-soaked cloths (10% solution) and placed in two easy-to-seal plastic body bags impervious to liquids, manufactured to VDI (Association of German Engineers) standards. Once the bags have been sealed with liquid-tight adhesive tape they must be decontaminated externally with a suitable disinfectant (see chapter on 'Disinfection'). Packed in this way, the body can be removed and placed in a coffin. The base of the wooden coffin must be covered with a sufficiently thick layer (at least 5 cm) of absorbent materials (sawdust, wood shavings, fleece etc.). The body should then be sent for cremation. A second autopsy should be avoided if possible, or performed under appropriate safety measures when the body is placed in the coffin.
Disposal management
The waste produced should if possible be disinfected as close as possible to the point at which it was generated. Items contaminated with pathogens which pursuant to Section 17 IfSG require special measures, and which cannot be safely decontaminated, should be disposed of together with the used and PPA in accordance with waste code 180103*.
For transport to the hazardous waste incineration (HWI) facility, pursuant to the ADR waste contaminated with L4 pathogens must always be packed in accordance with packing regulation P620 and labelled UN 2814. Since in practice no receptacles of a sufficient size are currently available, the waste may be packed in accordance with the multilateral agreement M315. The transport of infectious materials is essentially governed by the stipulations of the ADR for Class 6.2, Category A substances.
For the incineration of waste from health facilities which is contaminated with pathogens in risk group 4, no further special regulations are necessary at HWI plants above and beyond the above. For the operator of the HWI facility, no obligation to dispose arises from existing contracts or tender obligations for this waste code.
For pathogens that do not fall under ADR Class 6.2 Cat. A Infectious Substances, or for which only cultures fall under Cat. A , hospital waste can be packed like other infectious waste in accordance with packing regulation UN 3291, and disposed of in accordance with communication18 of the Federal/State Working Group on Waste (LAGA) – Guidelines on the disposal of waste from health service facilities.
Waste whose collection and disposal is not subject to any special requirements as far as infection prevention is concerned can be disposed of in accordance with AS 18 01 04.